Working with Industry
Contract Research and Services
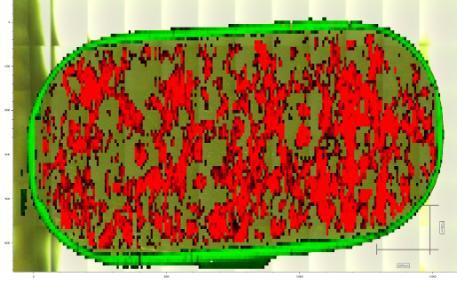
The Centre houses an impressive range of sophisticated analytical instrumentation allowing us to offer a broad spectrum of solutions to resolve any problem. Our dedicated analytical experts have many years experience working with a range of techniques allowing them to be innovative when developing products and troubleshooting commercial challenges. The Centre has an excellent record of delivering projects on time and in full, at a competitive price.
We pride ourselves on our ability to solve problems through analytical science, no matter how small or large your challenge, please contact us to discuss how we can help.

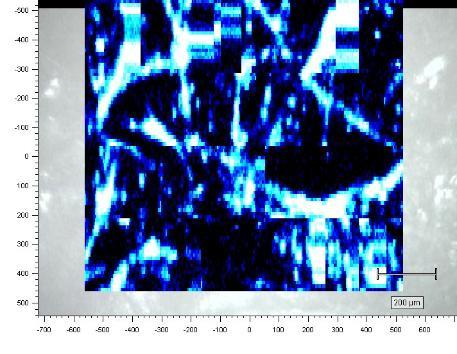
Renishaw InVia Raman Microscope