Technology opportunity novel abuse resistant controlled drug delivery
Scientists at the University of Bradford have invented a novel polymer matrix system that can be used to prepare novel abus resistant drug delivery systems.
The problem
Prescription opioid analgesics are an important component of modern pain management. Abuse and misuse of these products, however, have created a serious and growing public health problem, particularly in the USA. The FDA has worked to address this problem while ensuring that patients in pain have appropriate access to opioid analgesics. One potentially important step towards the goal of creating safer opioid analgesics has been the development of opioids that are formulated to deter abuse. FDA considers the development of these products a high public health priority.
The solution
Scientists at the Centre for Pharmaceutical Engineering Science (CPES) at the UoB have invented a novel polymer matrix system comprising of biocompatible, biodegradable, GRAS status, pharmaceutically acceptable, readily available and inexpensive materials that can be used to prepare novel drug delivery systems using readily scalable and efficient hot melt extrusion or micro moulding processing techniques. When the matrix is prepared with drug substance, the resultant drug product will have the desired quality attributes required by the
FDA for Abuse Resistant Systems (Tier 1) and have been shown to comply with the FDA’s required product characteristics in terms of ability to crush and ease of extraction (see preliminary data below). The prototype formulations also release the full drug loading over a 12hr period and the rate of release can be modified by changing the blend of matrix components. In addition, the novel polymer matrix can be formed into microneedles for controlled transdermal drug delivery using micro injection moulding processing techniques.
As recommended by the FDA Guidance for Industry-Abuse Deterrent Opioids- Evaluation and Labelling, CPES ARS formulations were evaluated using different test conditions for in-vitro drug release studies, preliminary data summarised in table below.

CPES Abuse Resistant formulation-Laboratory Extraction Studies
CPES ARS Formulation (10mg oxycodone)
| Abuse simulation tests |
BATCH 1 (% drug release) |
BATCH 2 (% drug release) |
|---|---|---|
| Release of drug in 450ml of disso media (0.1N HCl) in 15mins | 13.67% | 13.33% |
| Release of drug in 450ml disso media (0.1N HCl) in 15mins pretreated with 40% alcohol | 16.5% | 18.7% |
| Release of drug in 450ml disso media (0.1N HCl) in 15mins pretreated with vegetable cooking oil | 6.6% | 10.5% |
| Release of drug in 5ml water boiled and cooled, filtered | 9.23% | 12.8% |
| Release of drug in 450ml disso (H2O) by 12 hours | 100% | 100% |
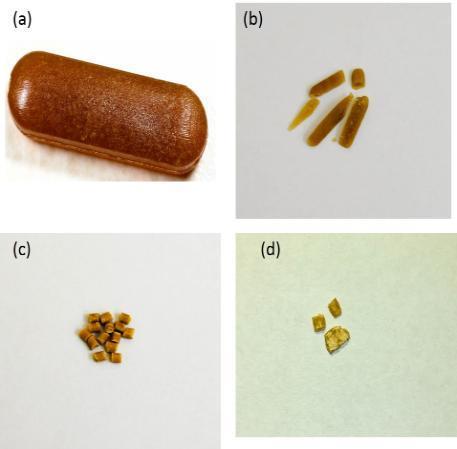
In addition, both injection moulded tablets [(a) before: (b) after] and extruded pellets.
[(c) before: (d) after] showed very high resistance to crushing in the range of 300-350N.
Opportunity
The University of Bradford is seeking industrial partners for co-development of the technology or product specific development using the technology with the view to out-licensing the technology to the industrial partner.
IP Status
Proof of concept data has been generated
Application
- Controlled release abuse resistant systems for opioid or controlled substance drug delivery
- Biodegradable microneedles for controlled transdermal drug delivery
Benefits
- An abuse resistant controlled release formulation designed for opioid drug delivery that can be manufactured into a convenient dosage form using readily scalable processing techniques
- The release profile can be modified and optimized by changing the composition of the matrix
- The novel polymer matrix is prepared from biocompatible, biodegradable, GRAS status, pharmaceutical acceptable, readily available and inexpensive materials
- The polymer matrix can be formed into microneedles for controlled transdermal drug delivery using readily scalable processing techniques
