Drug development pipeline at the Institute of Cancer Therapeutics
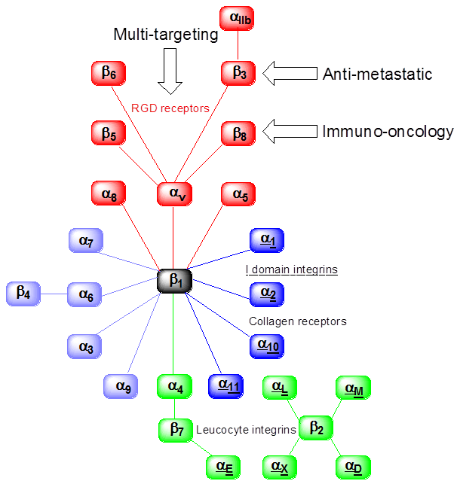
Researchers at the Institute of Cancer Therapeutics (ICT) specialise in the discovery and development of novel cancer therapeutics from target identification through to full preclinical validation.
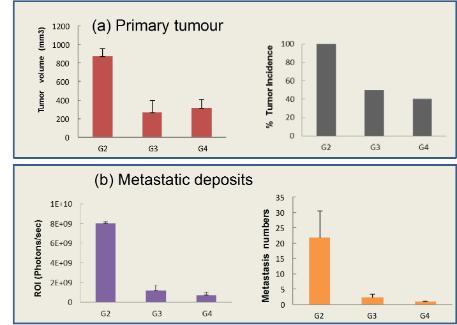
We understand that for our research to have an impact it needs to be translated into new and more effective medicines for cancer patients. To achieve this key objective the ICT seeks external collaboration and investment for the progression of a number of exciting research programs in order to advance them through into clinical trials and beyond. The ICT has an excellent track record of attracting investment for its research programs and has an exciting drug development pipeline.